Methodology
On January 30th, 2020, the WHO declared the SARS-CoV-2 outbreak a public health emergency of international concern (PHEIC), which implies that an adequate answer to combat or control this viral infection is urgently required and should rapidly become available. Worldwide vaccine development efforts have been launched but, once available and ready for distribution and administration, will take time to elicit an appropriate immune response against the virus. In addition, it is currently uncertain whether there will be adequate first/one-shot immunity. Moreover, based on our knowledge of other coronaviruses and other RNA viruses, the virus will almost certainly adapt to evade vaccine-induced immune responses.
As indicated by the previous outbreaks involving SARS-CoV (in 2003) and MERS-CoV (in 2012), and now with the current SARS-CoV-2 epidemic (2020), it is only a matter of time for the next zoonotic coronavirus to emerge. It is thus of key importance not only to invest in a therapy for the current epidemic, but also to be prepared for future (local or pandemic) coronavirus outbreaks. Therefore, we propose three multi-paced approaches reflecting a short, intermediate and long-term strategy to fight this infection.
As part of the answer to quickly respond to current and future outbreaks, it will be key to have an effective antiviral therapy at hand. However, as it takes many years to develop such novel, specific and potent anti-coronavirus drug, repurposing existing drugs that are already on the market (or have reached advanced stages) is considered to be an efficient short-term strategy to treat novel infections. Indeed, preliminary encouraging results have recently become available from testing a few FDA-approved drugs, of which some also show modest activity against SARS-CoV or MERS-CoV. These compounds also showed moderate activity against the SARS-CoV-2 (1). During the course of the SCORE project, we will (re-)evaluate several drugs approved for human use in one or more regions of the world and in-house developed compound libraries. In addition, as it is a proven valuable strategy in developing better antiviral treatments, we will also explore the combined potency of two or more molecules that have moderate potency on their own. The potency of such combinations may be sufficient to have an impact in SARS-CoV-2 -infected humans. In some cases, combinations may show synergistic antiviral effects. Likewise, combinations resulting in antagonistic effects (e.g. ribavirin and some pyrimidine nucleoside analogues) should be identified and excluded.
Because of the prior SARS and MERS outbreaks, ample experience on coronaviruses is present within the SCORE consortium. Several partners have collaborated in coronavirus research since the days of the SARS outbreak. Most partners collaborated in the FP7 SILVER project (on antiviral drug development for emerging RNA viruses) when MERS-CoV emerged in 2012, and united their efforts in a specific MERS-CoV drug discovery pipeline within that project (see below). These already more advanced molecules will be the base of the second, intermediate-term strategy.
As mentioned above, it is evident that 2019-nCoV will not be the last public health threat to emerge from the coronavirus family. Therefore, it will be of utmost importance to have potent inhibitors at hand that possess broad-spectrum anti-coronavirus activity, i.e. that will inhibit the replication of all known coronaviruses (pan-coronavirus antiviral compounds). This will allow us to respond more swiftly and with enhanced precision when the next coronavirus crosses the species barrier from animals to humans. Potent coronavirus antivirals will not only help to save the life of those that are severely ill, prophylactic treatment of contacts of such infected persons may also help to prevent further transmission. Having potent and pan-coronavirus inhibitors available will also be an essential tool to curb outbreaks in the very early stages of emergence of the next coronavirus. This is a major difference with a vaccine, the construction of which can only be initiated at the time the pathogen is at hand, has been identified and characterized. Therefore, as part of its longer-term strategy, the SCORE consortium will engage in high-throughput screens using phenotypic assays (virus-cell culture infection models) to identify novel, selective, and highly potent classes of pan-coronavirus inhibitors with a high barrier towards developing viral drug resistance.
In the past, we and others observed that, for different viruses, molecules with very different chemical structures may have the same mechanism of action. One example is the “hotspot” that we identified in the pestiviral RNA‐dependent RNA polymerase, which is targeted by at least 5 different classes of molecules (2). Within this context, we will also use these large-scale screens to map all potential antiviral drug ‘targets’ in the coronavirus genome (generating a coronavirus ‘antivirome’). This concept shows parallels with the mapping of the druggable genome of the malaria parasite. It can be extremely useful to reveal such hotspots for inhibition and to identify compounds with different mechanisms of action. In addition, knowledge on the target of a drug can also expedite drug development.
Today, highly potent drugs are available for the treatment of infections with HIV (~30 approved drugs), herpesviruses, HBV, and HCV. Potent drugs are also available against influenza virus, although still more and more potent drugs are needed. What potent antiviral drugs can do, is perhaps best reflected by the drugs that have recently become available to treat HCV infection. Very well-tolerated antiviral therapy now enables curing ~99% of chronically infected HCV patients within about 2 months. Zoonotic coronaviruses cause acute but (in general) relatively slowly progressing and self-limiting infections, which implies that the infection often can be cleared by a normal immune response. Drugs that reduce viral replication will help to reduce symptoms in diseased patients and buy time for the immune system to mount a full-scale protective immune response. Therefore, it should be possible to prevent severe illness or death by a short-term treatment with a potent antiviral molecule. The antiviral drug experts of the SCORE consortium strongly believe that it will be possible to develop such potent and selective antiviral drugs for SARS-CoV-2, provided that our efforts are of a sufficiently large scale, focused, driven by the most appropriate actors, and broadly supported by the right people. We are convinced that, in that scenario, significant progress and success are inevitable.Supported by a human-centered, systems design thinking approach to obtain insights in the joint impact of specific, combined or diverse antiviral interventions at the last mile, a high degree of complementarity with SCORE actions can be attained. At the same time, limitations and opportunities embedded in the last mile contextual situations, can be assessed to be taken into account at the very early R&D stages that the SCORE project is basically about. In this way, we are convinced that significant buy-in, progress and success are inevitable.
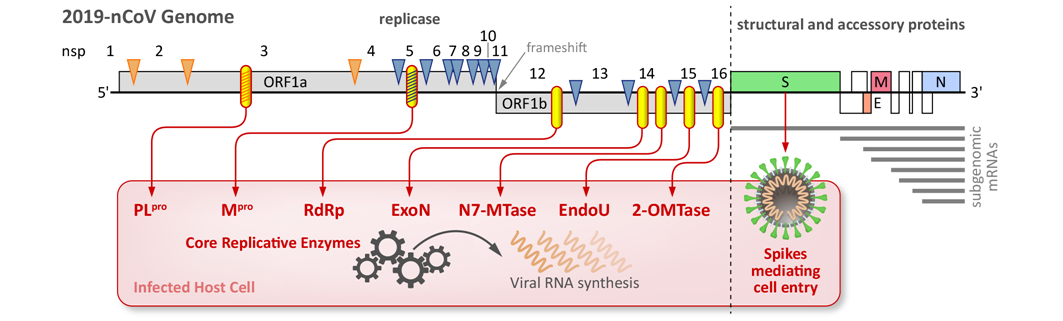
Overview of the SARS-CoV-2 genome and the primary enzymatic and entry functions targeted for drug development in the SCORE project

.jpg)
%20(Phone).jpg)
%20(Phone).jpg)
%20(Phone).jpg)
.jpg)
.jpg)