SCORE
The COVID-19 pandemic and the SCORE consortium (1-Apr-2020 to 1-Oct-2022)
In early 2020, SARS-coronavirus-2 (SARS-CoV-2) took the world by surprise, despite prior warnings in the form of the first SARS-CoV outbreak (2002), the emergence of MERS-CoV (2012), and numerous indications that – with little or no adaptation - animal coronaviruses may infect human cells. Thirty months later, the COVID-19 pandemic has resulted in over 617 million confirmed cases and more than 6.5 million deaths, with the true death toll estimated to be probably 2 to 3 times higher than this number.
Immediately after the start of the pandemic, major efforts were launched to rapidly produce effective vaccines, which indeed became available at unprecedented speed and contributed importantly to reduce the impact of COVID-19 worldwide. However, from the beginning, it was also clear that additional tools would be needed to counter this virus, which rapidly began to escape the immunity induced by both vaccination and natural infection on the basis of the evolutionary plasticity of its Spike protein.
Antiviral drugs arguably are the most important additional asset in the pandemic preparedness toolbox, as they can be used both therapeutically (to treat COVID-19 patients) and prophylactically, to protect people coming in direct contact with infected individuals (e.g., health care workers, frontline workers and teachers). By reducing virus shedding by infected patients, their direct contacts could be protected as well. Sufficiently potent antiviral drug treatment should help to reduce the R0 to below 1, which would be an essential for worldwide efforts to curb the pandemic.
Antiviral drugs can be targeted at coronavirus functions that are known to evolve less rapidly than the Spike protein. The conserved nature of such functions also increases the chance that such drugs may inhibit the replication of a broader group of coronaviruses, and may thus also contribute to pandemic preparedness for future coronavirus outbreaks. Ideally, broad-spectrum antiviral drug combinations would be developed, which could be used to immediately contain local virus outbreaks. Such highly potent and safe directly-acting antiviral drugs were already available for the treatment of a number of chronic viral infections like HIV and hepatitis C. Combinations of drugs with different modes of action, provided in daily pills, can suppress or even cure these viral infections, demonstrating how successful and life-saving antiviral drug treatment can be.
It was against this background that the SCORE consortium (for SWIFT CORONAVIRUS THERAPEUTICS RESPONSE) was established in February 2020, to respond to one of the first EU calls for research proposals to counter the rapidly spreading SARS-CoV-2. The early realization that potent small-molecule antiviral drugs might be crucial to fully control this rapidly evolving RNA virus brough together eight academic and industrial partners (see table below), with extensive expertise in coronavirus research and antiviral drug development. In part the consortium was built on pre-existing collaborations that went back to the response to the SARS-CoV outbreak of 2002-2003 (EU projects SARS-DTV and VIZIER) and the emergence of MERS-CoV (EU projects SILVER, EUVIRNA and ANTIVIRALS).
Overview of SCORE partners
| Participant No. | Participant organisation name | City | Country |
| 1 (Coordinator) | Leids Universitair Medisch Centrum (LUMC) | Leiden | The Netherlands |
| 2 | Université d'Aix Marseille (AMU) | Marseille | France |
| 3 | Katholieke Universiteit Leuven (KU Leuven) | Leuven | Belgium |
| 4 | Universiteit Utrecht (UU) | Utrecht | The Netherlands |
| 5 | Eidgenössisches Departement des Innern (EDI-IVI) | Bern | Switzerland |
| 6 | Universität zu Lübeck (UzL) | Lübeck | Germany |
| 7 | Helmholtz-Zentrum für Infektionsforschung (HZI) | Braunschweig | Germany |
| 8 | Janssen Pharmaceutica (JP) | Beerse | Belgium |
The SCORE drug development pipeline
The pre-pandemic collaborative coronavirus studies of the SCORE partners covered a number of areas. Among them were structure-function analysis of a range of coronavirus nonstructural proteins and the Spike protein, studies of a range of (potentially druggable) virus-host interactions, structure-based drug design efforts, and many collaborations leading to the development of an extensive coronavirus toolbox and various compounds in (early) pre-clinical development. The latter were included in the SCORE drug development pipeline from the start, while other efforts focused on the screening of large chemical libraries, the targeted development of new or better inhibitors of specific viral enzymes, and the generation of SARS-CoV-2 specific molecular tools and assays. These tools included biochemical and biological assays, virus strains, reverse genetics systems, animal models and a range of protocols, and their development was facilitated by our vast experience with closely related coronaviruses. Their integration into a drug development pipeline is illustrated in the scheme below.
The SCORE consortium aimed to develop antiviral therapy to combat both SARS-CoV-2 specifically and coronaviruses in general. To that end the consortium uses not only a target-based approach to inhibit viral entry or replicative enzyme functions, but also high-throughput antiviral screens in cell culture-based SARS-CoV-2 infection models. The latter approach identified a number of robust antiviral hits that have been further optimized using a medicinal chemistry approach. During its first year alone, the SCORE research and development program yielded 21 peer-reviewed scientific publications, many of which appeared in leading journals (see https://www.score-cov.eu/Article/Publications for an overview of all SCORE publications thus far).
In the second year of the project, the obtained chemical compound hits were further developed to improve their activity. These new compounds have also been employed as part of our ongoing efforts to dissect coronavirus molecular biology and evolution, map SARS-CoV-2 drug targets at the molecular level, and uncover the Achilles’ heel(s) of viral replication. These studies may also prove essential to prepare for future pandemic encounters with newly emerging coronavirus threats.
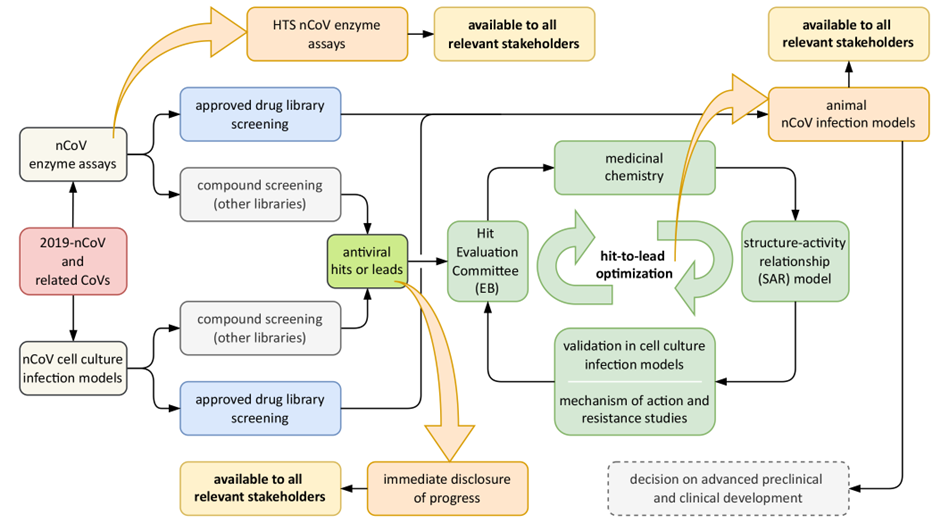
Overview of SCORE antiviral drug development pipeline, employing both cell culture-based screening and in vitro enzymatic assays to select hits/leads to be further optimized by medicinal chemistry.
Organization of SCORE research
The SCORE project was organized in 9 work packages (see Table below). Four WPs (1-4) focused on different approaches and viral targets to identify novel hits or improve existing ones. WP1 focused on chemical library screening, WP2 on nucleoside/tide analogues and the coronavirus RNA polymerase, WP3 developed viral protease inhibitors, and WP4 focused on the viral Spike protein and entry inhibitors. These WPs were supported by a SARS-CoV-2 toolbox that was continuously developed in WP5, while WP6 encompassed the development of animal models for compound testing. WP7 included the consortium’s communication and public outreach efforts, WP8 overall SCORE project management, while WP9 was specifically concerned with ethics.
Overview of SCORE work packages and work package leaders
| Work Package | Work Package Title | Lead Participant | Lead Participant Short Name |
| 1 | Library screening | 3 | KU LEUVEN |
| 2 | Nucleoside/tide analogues | 2 | AMU |
| 3 | Viral protease inhibitors | 6 | UzL |
| 4 | Entry inhibitors | 4 | UU |
| 5 | The SARS-CoV-2 toolbox | 1 | LUMC |
| 6 | Animal models | 5 | EDI-IVI |
| 7 | Communication and public outreach | 1 | LUMC |
| 8 | Management | 1 | LUMC |
| 9 | Ethies | 1 | LUMC |
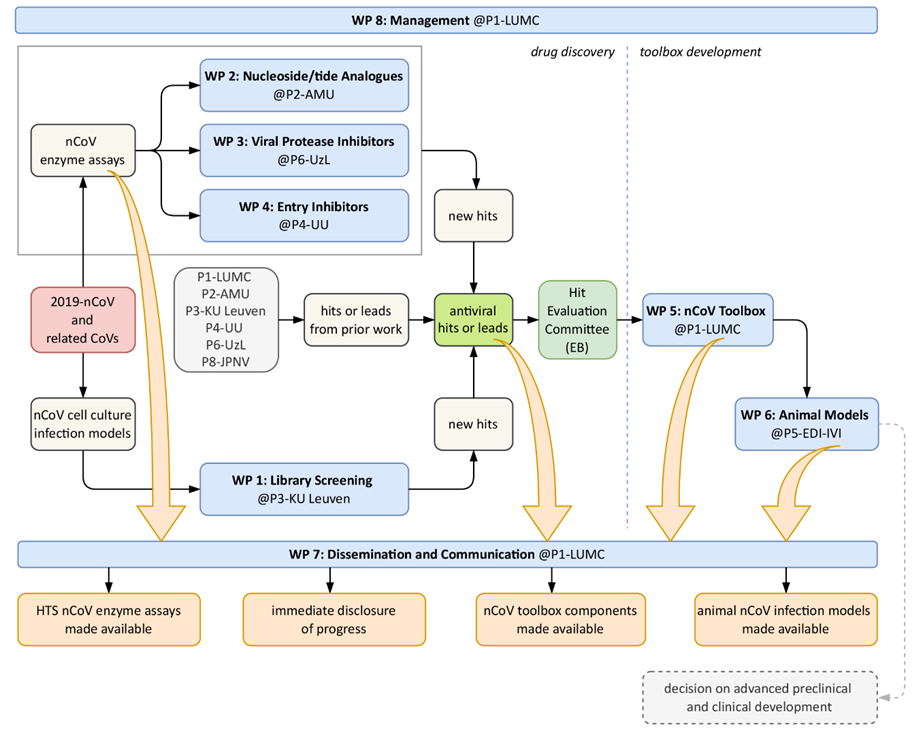
Workflow, output and connectivity of the tasks/work packages of the SCORE drug discovery pipeline
Products and knowledge developed within the SCORE consortium
The SCORE project has been highly productive, in terms of SARS-CoV-2 and coronavirus knowledge gained, SARS-CoV-2-specific tools and assays developed, and drug candidates identified and/or developed. Given its relatively short duration of 30 months (April 1, 2020, to September 30, 2022), and the many complications and restrictions encountered in these pandemic years, it is not surprising that several research lines will be continued beyond SCORE, either in the context of other collaborative projects or supported by new initiatives. Consequently, more SCORE publications are expected to appear in the months to come and will be added to our publication list. As a large part of SCORE’s output has already been published, we would like to refer to the same publications list for a complete overview of our achievements. A summary of main achievements, although unavoidably incomplete, is listed below.
SARS-CoV-2 and coronavirus knowledge gained
- Detailed information on the efficacy of ~9,5000 drugs considered for drug repurposing
- In-depth characterization of the structure and function of the coronavirus RNA polymerase complex (nsp12-nsp7-nsp8)
- Detailed biochemical characterization of the mechanism of action of all leading nucleoside analogue dug candidates
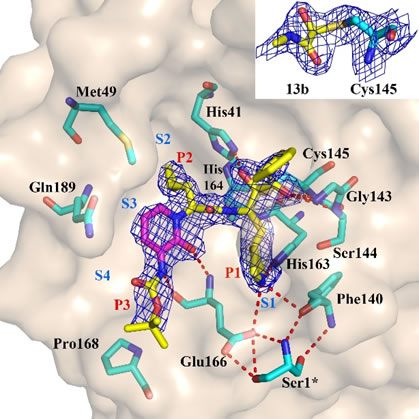
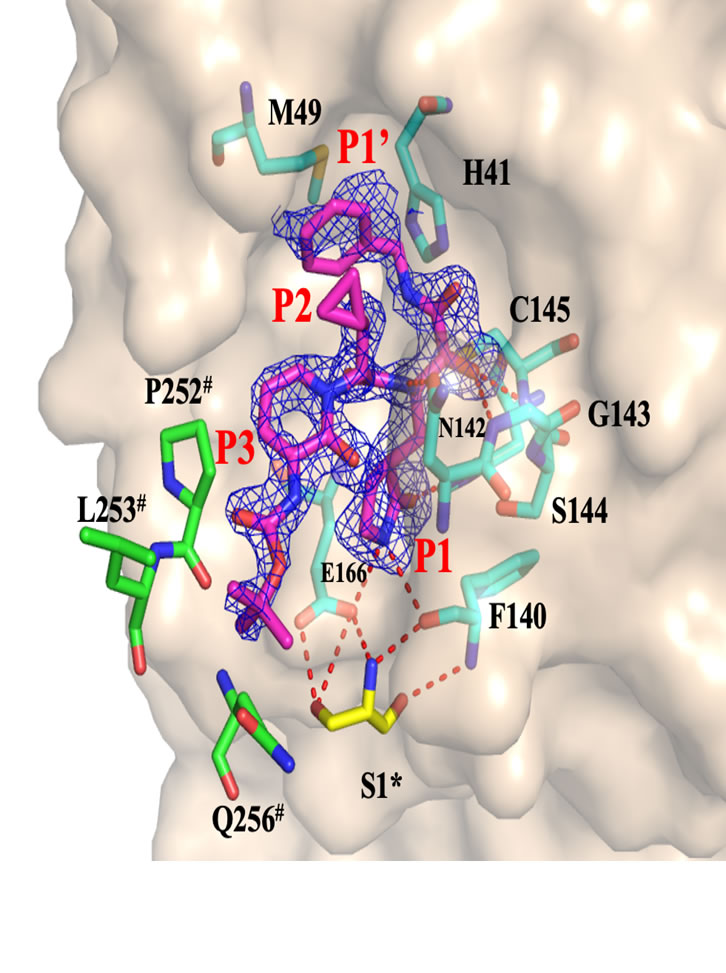
- A new area of chemical modifications has been developed with the application of macrocyclic nitriles and cyanohydrins as inhibitors of the SARS-CoV-2 Mpro".
- Detailed structure-function analysis of the N7-methyltransferase and exoribonuclease activities of nsp14
- In-depth characterization of SARS-CoV-2 main protease structure and function (nsp5)
- Characterization of inhibitory properties and antiviral activity of existing and novel α-ketoamide inhibitors of the coronavirus main protease (nsp5)
- Cryo EM visualization of coronavirus replication organelles and identification of an RNA export channel formed by nsp3
- Design and antiviral activity of nanobody-conjugated Heptad Repeat (HR) targeting peptides that inhibit the function of the SARS-CoV-2 Spike protein
- Introduction of an innovative system for coronavirus reverse genetics based on the use of transformation-associated recombination (TAR) in yeast
- Establishment and characterization of Syrian golden hamster and mouse models for SARS-CoV-2 infection
Highlights from the joint SCORE toolbox
- A complete set of fully characterized SARS-CoV-2 variants, made available to all partners as SARS-CoV-2 evolution progressed throughout the pandemi
- Pseudotyped viruses containing the Spike protein of SARS-CoV-2 variants for entry studies.
- Extensive set of (engineered) cell lines with desired characteristics, used for SARS-CoV-2 antiviral drug screening and investigation of SARS-CoV-2 molecular biology
- Protocols for a large number of antiviral screening assays developed using those cell lines, allowing characterization of viral protein functions, large scale screenings and detailed analyses of viral infection.
- Expression vectors and purified recombinant proteins for a large number SARS-CoV-2 enzymes (including RNA polymerase, proteases, exoribonuclease and methyl transferases) and the viral Spike protein
- Protocols for a large number of in vitro enzyme assays (including RNA polymerase, proteases, exoribonuclease and methyl transferases)
- Antibodies, nanobodies and antisera targeting a range of SARS-CoV-2 proteins
- Protocols for coronavirus reverse genetics including the innovative transformation-associated recombination (TAR) approach in yeast, to rapidly engineer and launch virus mutants or variants for a range of comparative or structure-function studies
- Models for SARS-CoV-2 infection in HAE cultures, Syrian golden hamsters and (transgenic) mice
SCORE reagents and protocols are available to the international research community upon request.
Products developed within the SCORE consortium and tested in animal models:
- The antiviral drug 13b-K, an inhibitor of the SARS-CoV-2 main protease
- Compound series 06, an antiviral drug specifically inhibiting SARS-CoV-2
- Compound series 08, a broad-spectrum coronavirus inhibitor which is further developed within the CARE consortium
- HR targeting entry inhibitors for SARS-CoV-2
- SAR405, an inhibitor of host factor VPS34 (a class III PI3K).
- Suramin, a repurposed compound that inhibits SARS-CoV-2 replication and was used in combination with a new application method
The secret of SCORE’s success?
The SCORE partnership was uniquely shaped to dampen the economical and public-health threats of present and future emerging coronaviruses. Due to its intersectoral nature, the SCORE consortium incorporated highly complementary expertise to explore, deliver, and transfer anti-coronavirus drug candidates and the associated high-end research to our societies.
The SCORE project has been operational from April 2020 up to October 2022, and covered the period during which rapidly evolving SARS-CoV-2 variants continued to appear and replace each other worldwide. The eight SCORE partners worked during this challenging period, with national lock-down periods, infected scientists and severe shortages in lab equipment, and lack of direct personal interactions and possibilities to recruit new scientists due to international travel restrictions.
Due to the scientific expertise, motivation, and highly successful collaboration the SCORE consortium succeeded to develop six different antiviral products until technological readiness level TRL 6 (proof of concept studies in animal models), established an extensive toolbox of which the products, reagents and technologies were readily shared between all partners, and the publication of 33 scientific articles, with another 7 submitted at this moment (September 2022). Another 10 SCORE publications are expected to be submitted in the coming year, and the latest publication update is always available under: https://www.score-cov.eu/Article/Publications. Another very important asset has been the training of new SCORE scientific staff in developing antiviral drugs against an emerging pathogenic virus, under very difficult conditions. These people will represent an important reservoir of veteran pandemic preparedness scientists, with the motivation and training to tackle any new pandemic.
What was the basis for the success of the relatively small SCORE consortium? …..
- A history of productive pre-existing collaborations in previous European consortia
- A team of academic partners with highly complementary scientific expertise
- Excellent understanding of each other’s scientific interests and technological capabilities
- Easy sharing of toolbox instruments, products and technologies between partners
- A small and flexible network, with the ability to quickly adapt to newly arising problems
- Regular virtual meetings and presentations
- An informal and efficient management structure, facilitated by the modest size of the SCORE consortium
- A strongly committed, experienced and highly knowledgeable Scientific Advisory Board
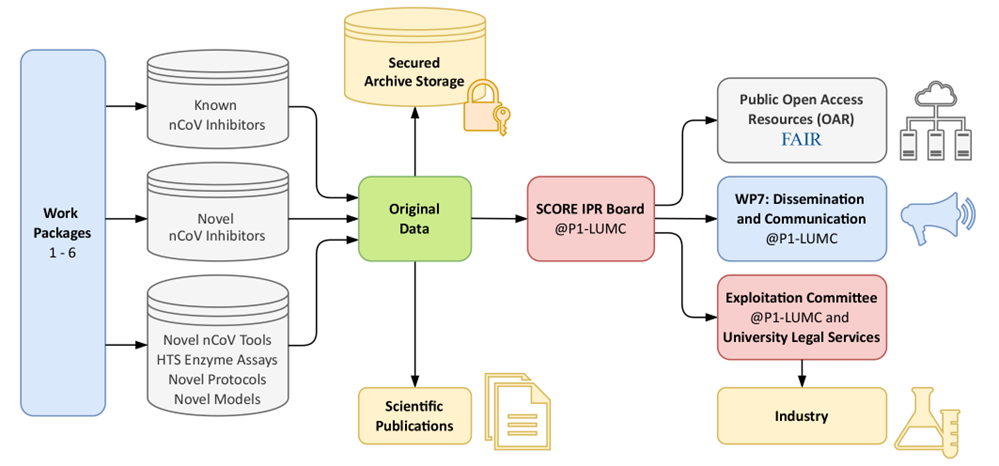
Outline of SCORE management and dissemination.
Outlook
Despite the success of SCORE, and many other worldwide research activities and projects targeting SARS-CoV-2, it is clear that the (potential) impact of a viral pandemic on society and economy had been underestimated for several decades. The COVID-19 pandemic overwhelmed health systems when it turned out that – unlike SARS-CoV in 2002/2003 – this rapidly spreading (and evolving) respiratory viral agent could not be contained using basic control measures like isolation and quarantine. Antiviral drugs, if available, can have a major impact on the early control of a local outbreak of a newly emerging virus, whether a coronavirus or a member of one of the other high-risk virus families that have a record of successful zoonotic transmission events.
The clear lesson of the COVID-19 pandemic is that vaccine and drug development should move forward, especially in ‘peace time’ and supported by relatively modest but continuous investments. This will allow the steady development of basic virus knowledge and better technological starting points to counter infections with a range of high-risk virus families. It would be important to avoid interruptions of successful collaborative antiviral research due to funding gaps. In this sense, the COVID-19 pandemic has revealed a strong need for the development of a roadmap for the design, evaluation and production of (broad-spectrum) antiviral drugs. All members of the SCORE consortium remain eager to contribute to this goal on the basis of there established expertise in the area of virology, biochemistry, structural biology and/or medicinal chemistry.
Further information
For further information on the SCORE project, please contact
- Dr. Ed Schmidt (SCORE project manager), e.d.l.schmidt@lumc.nl, or
- Prof. Eric Snijder (SCORE coordinator), e.j.snijder@lumc.nl.
The SCORE project was funded under the European Union’s Horizon 2020 Research and Innovation program, grant agreement 101003627.